
Four Ways to Keep Your Cleanroom Contaminant-Free
Contamination pose a serious threat to the safety and integrity of compounded pharmaceutical products. USP <797> outlines the steps that need to be taken to maintain a safe, contaminant-free cleanroom.
Preventative action and early detection of possible sources of contaminants can help pharmacies stay compliant with FDA regulations.
The following four actions are a good starting place for keeping your compounding pharmacy clean:
1. Identify sources of contamination
The first step to maintaining a clean cleanroom is identifying potential sources of contamination. Microorganisms can survive and grow on humans and in water, which are considered to be primary sources of contamination. Contaminants are transferred from humans and water to secondary sources, like air and surfaces, where they survive but do not grow.
- People
- Approximately 1,000 species of microorganisms live on the human skin, including bacteria like Staphylococcus spp and Micrococcus spp. Humans shed these microorganisms into the air, water sources and cleanroom surfaces. Humans are also a source of non-viable contamination like dirt and other particulate matter.
- Water
- Water is used in cleanrooms as a drug ingredient, cleaning agent, disinfectant and steam supply. Fungi and Gram-negative bacteria are commonly found in water.
- Air
- Contaminants in the air are typically bacteria found in dust particles or human skin flakes.
- Surfaces
- Contaminants are found on surfaces around cleanrooms, including equipment, non-sterile items and packaging materials. Bacillus and fungi are commonly found on cardboard.
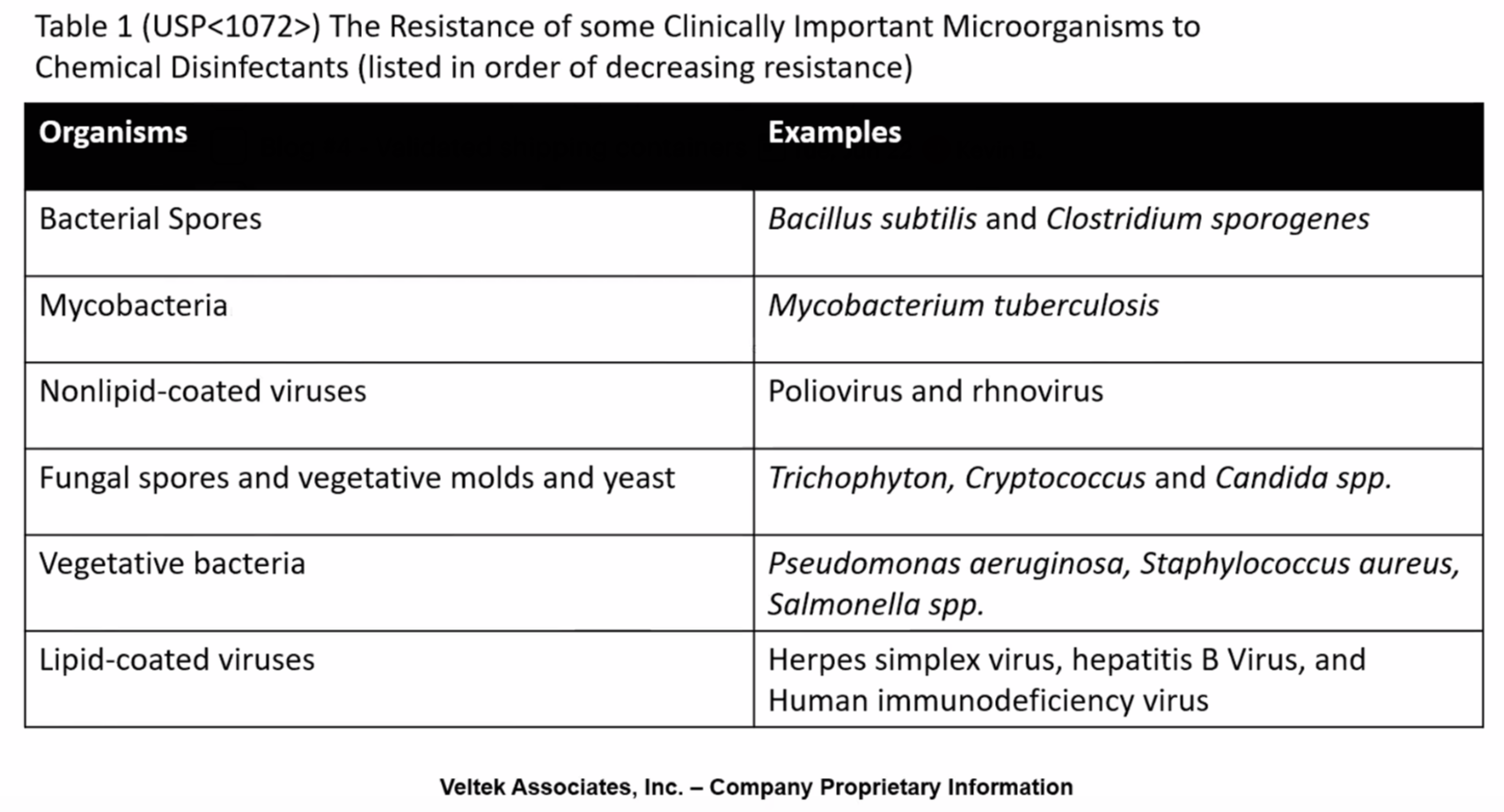
Failures in engineering controls and incorrect aseptic techniques can also lead to cleanroom contamination.
2. Implement proper cleaning, sanitizing and disinfecting procedures
Following a regular cleaning schedule will help prevent contamination in cleanroom pharmacies. Each pharmacy should develop their own cleaning scheduled and protocols based on USP guidelines.
- Decontamination
- Removal of microorganisms by disinfection or sterilization
- Typically done daily, weekly and monthly with disinfectants
- Disinfection
- Destroying or removing vegetative forms of harmful microorganisms
- Typically done daily, weekly and monthly with disinfectants
- Deactivation
- Safely removing and destroying hazardous drugs from the compounding surface
- Typically done after compounding
3. Follow proper gowning and hand-hygiene procedures
Proper gowning and hand hygiene will help eliminate human-borne contaminants. Each compounding pharmacy should develop and maintain SOPs based on USP guidelines for gowning and hand hygiene.
The following gowning and hand hygiene procedures are recommended:
- Use aseptically packaged garments made specifically for cleanrooms
- Garments include hoods, face masks, coveralls, bunny suits, shoe covers and boot covers
- Wash hands thoroughly before gowning
- Remove any items that can introduce contamination
- Examples: jewelry, watches, false nails, makeup
- Perform garbing qualification
- Leave the cleanroom and re-gown if garments are suspected of being compromised
- Do not reuse garments
4. Conduct environmental monitoring
Viable particles contain living microorganisms, are able to germinate and impact the sterility of compounded products. Fungi, bacteria, viruses and spores are all viable particles. Non-viable particles are organic particles in the air that do not contain living microorganisms but can transport them. Particulates like dust, dirt, pollen, fibers, rust and chemical compounds are considered to be non-viable particles.
It is critical to monitor cleanroom air and surfaces for both viable and non-viable particles, as they can negatively affect the sterility of compounded products. Each pharmacy should develop a schedule and SOPs for environmental monitoring based on USP <797> guidelines.
- Viable particle monitoring
- Methods: Active air sampling (volumetric air sampler), passive air sampling (settle plates), surface samples (contact plates and swabs) and personnel samples (finger, sleeve and gown plates).
- Monitoring locations and frequency: The location and frequency of monitoring depends on the size and layout of the cleanroom, as well as the type of compounding taking place. It is typically done during compounding.
- Non-viable particle monitoring
- Methods: Particle counters (fixed or portable).
- Monitoring locations and frequency: Fixed particle counters provide continuous monitoring, while portable counters are used for less frequent monitoring. Both monitors should be located in critical areas.
Stay compliant with DiNovo
Proper environmental monitoring, preventative actions and process improvements are critical for staying compliant with FDA regulations. DiNovo Pharmacy and Packing Provisions is here to help. We sell all environmental monitoring products, including garbing materials, sample plates, air monitoring devices, cleaning supplies and more.
We can also help pharmacies develop SOPs for cleaning, garbing and environmental monitoring. Reach out to our sales team at. sales@dinovomed.com to view the recording of our Environmental Monitoring webinar with Veltek Associates.
About DiNovo
Oversight of the compounding industry is increasing. At DiNovo, we put patient safety first. We provide supplies for 503A and 503B pharmacies, including supplies for packing and shipping, cleaning and disinfecting, and compounding. Contact us today or download our compliance checklist to learn more.
.png?width=500&height=153&name=DiNovo%20Full%20Logo%20-%20Both%20Tags%20(500x153).png)